BACKGROUND
Our lab interests are focused on the mechanisms by which transcriptional, epigenetic and signal transduction factors regulate the fate of pluripotent and neural stem cells. Embryonic Stem (ES) cells are derived from pre-implantation embryos and share two unique properties: the ability to be grown indefinitely in culture (self-renewal) and the ability to differentiate into any cell type of an organism (pluripotency). In the last decade, the advent of somatic cell reprogramming technology allowed the production of induced Pluripotent Stem cells (iPS) that revolutionized the modelling of human diseases and the expansion of regenerative medicine. Among other, regeneration of the neural tissue is of paramount importance in order to fight neurodegenerative diseases. For that aim, the advent of patient’s iPSC derived neuronal cultures fulfils the need for more biologically faithful and clinically relevant models, especially on a personalized basis.
Our recent research projects are:
1. Identification and characterization of novel microRNAs that regulate ES cell fate decisions
Using the Ion Torrent platform (IMBB) we performed deep sequencing of miRNA expressed in mouse ESCs (mESCs) and differentiated embryoid bodies (EBs). Among differentially expressed miRNAs we characterized four novel miRNAs that are involved in ES cell differentiation choices acting as regulators of the TGF/Activin /BMP4 signaling pathway.
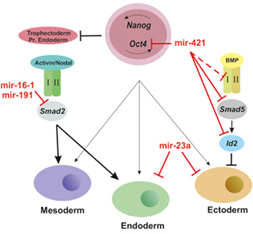
FIGURE: MicroRNAs for Mouse Embryonic Stem Cell Fate Decision through Regulation of TGF-β Signaling (C.Hadjimichael)
2. The role of Promyelocytic Leukemia Protein (PML) in Embryonic and Cancer Stem cells (A.Vogiatzoglou)
Promyelocytic leukemia protein (PML), the main constituent of PML nuclear bodies, regulates multiple cell functions including the stem cell properties in different tissues (hematopoietic system, nervous system and mammary gland) in normal and pathological conditions.
2a. We have shown that PML contributes to Embryonic Stem cell (ESC) self-renewal maintenance by controlling cell-cycle progression and sustaining the expression of crucial pluripotency factors. Transcriptomic analysis and gain- or loss-of-function approaches showed that PML-deficient ESC exhibit morphological, metabolic, and growth properties distinct to naive and closer to the primed pluripotent state. During differentiation of embryoid bodies, PML influences cell-fate decisions between mesoderm and endoderm by controlling the expression of Tbx3. PML loss compromises the reprogramming ability of embryonic fibroblasts to induced pluripotent stem cells by inhibiting the transforming growth factor β pathway at the very early stages. Collectively, these results designate PML as a member of the regulatory network for ESC naive pluripotency and somatic cell reprogramming.
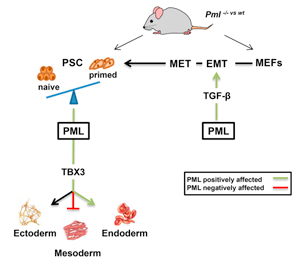
FIGURE: PML Is an Essential Regulator of Stem Cell Pluripotency and Somatic Cell Reprogramming (C.Hadjimichael)
2b. PML and Breast Cancer (A. Vogiatzoglou in collaboration with J.Papamatheakis)
In collaboration with J.Papamatheakis we study PML-dependent molecular signatures associated with tumor –suppressing or promoting effects in breast cancer. We have recently shown that PMLIV regulates cell cycle progression, stemness and stress response of breast cancer cell lines in a subtype-specific way, via multiple regulatory pathways. Among them, the FOXM1 transcription is a major PML target. Currently, we extend those studies using PML knock down setups to study the growth, invasion, gene expression profiles and in vivo tumor growth and metastatic ability in immunosuppressed NOD/SCID mice. We designed experiments to check the interplay of PML with wt- or mutant-p53 role in determining the effect of the former in cancer biology and the role of PML in the regulation of secreted factors that affect in vivo tumor behavior. By integrating the above via bioinformatics analysis, we identify molecular targets and mediators that affect cancer cell proliferation, stemness and invasion with the aim to develop new useful biomarkers and drug targets.
3. The role of Aminoterminal Enhancer of Split (AES) in ESC
Groucho related gene 5 (GRG5) is a multifunctional protein that has been implicated in late embryonic and postnatal mouse development. We have shown that ablation of GRG5 deregulates the Embryonic Stem Cell (ESC) pluripotent state whereas its overexpression leads to enhanced self-renewal and acquisition of cancer cell-like properties. The malignant characteristics of teratomas generated by ESCs that overexpress GRG5 reveal its pro-oncogenic potential. Furthermore, transcriptomic analysis and cell differentiation approaches underline GRG5 as a multifaceted signaling regulator that represses mesendodermal-related genes. When ESCs exit pluripotency, GRG5 promotes neuroectodermal specification via Wnt and BMP signaling suppression. Moreover, GRG5 promotes the neuronal reprogramming of fibroblasts and maintains the self-renewal of Neural Stem Cells (NSCs) by sustaining the activity of Notch/Hes and Stat3 signaling pathways. In summary, our results demonstrate that GRG5 has pleiotropic roles in stem cell biology functioning as a stemness factor and a neural fate specifier.
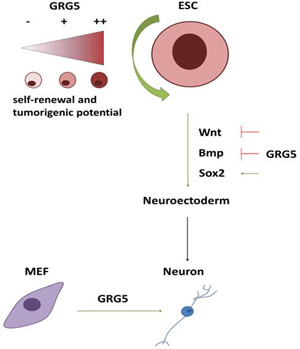
FIGURE: GRG5 is a self-renewal factor with decisive role in neural fate decisions (K.Chanoumidou)
4. Regulation of neuroregeneration using neural stem cells (NSC) derived from pluripotent stem cells) (Sirago Spanou)
This is a collaboration with the laboratories of Th. Kalogeropoulou (NRI) and A. Gravanis and I. Charalambopoulos (Medical School, IMBB). The effects of synthetic Micro Neuro Trophins (MNTs) on the self-renewal and differentiation of Neural Stem Cells (NSC) derived from pluripotent stem cells will be investigated. The goal is to develop an experimental system for modeling neurodegenerative diseases such as Alzheimers (AD). MNTs will be tested for their neuroprotective activity and the ability to alleviate experimentally induced neurodegeneration. Moreover we will examine the role of Pro-Myelocytic Leukemia (PML) protein in NSC functions and neurodegeneration For this purpose we will employ NSC that do not express PML (PML KD) and a cross of the 5XFAD mouse (an experimental model of familial AD) with the PML KO mouse. By this methodology we expect to test whether PML is involved and by what mechanism in the manifestation of the disease.




