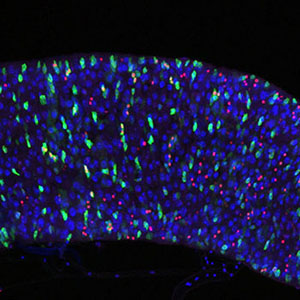
We are interested in how different transcription factors and chromatin regulators provide and safeguard stem cell identity in development and how their deregulation can lead to disease. A main cell-type under study in our lab is neural stem cells, called neuroblasts (NBs) in Drosophila, and how they behave and function in different developmental stages. NBs are born in early embryogenesis and divide asymmetrically throughout the juvenile stages of the animal (embryo, larva) to generate the entire central nervous system.
ASC proneural transcription factors in embryonic neurogenesis
When neuroblasts are born in early embryogenesis, the neuroectoderm expresses the proneural TFs, Achaete, Scute, Lethal of scute and Asense (the ASC proteins). This family of transcriptional activators promotes NB fate acquisition. At the same time, lateral inhibition via Notch signaling antagonizes NB fate. To elucidate the global chromatin changes that underlie NB specification, we employ genetic perturbations followed by genomic profiling for TF chromatin occupancy, histone modifications and transcriptional output to reveal regulatory networks that drive and sustain neural stem cell identity. [More]
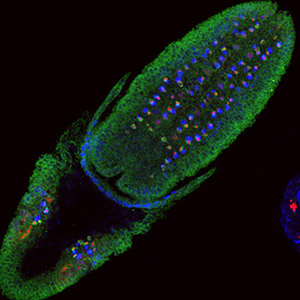
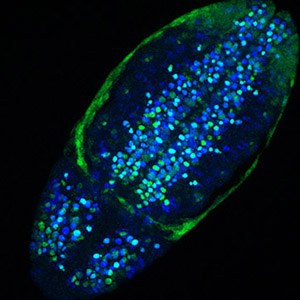
Neural precursor tumorigenesis by persistent Notch signaling
Notch signalling must be tightly spatiotemporally regulated, or else it leads to pathologies. Larval NB lineages with aberrantly activated Notch in the NBs' progeny cause primary tumours, which become malignant when allografted into the abdomens of adult hosts, leading to premature death of the recipient. [More]
Acquisition of cell fates in the Drosophila gut
In the digestive system, the same proneural TFs and Notch signalling are redeployed in several successive instances of development and adult life. As in the nervous system, their role is decisive in the developmental trajectories followed by progenitor cells. The major differentiated cell types of the gut are the absorptive enterocytes (ECs) and the hormone-producing enteroendocrine cells (EEs). Proneural protein activity is needed for early steps in EE differentiation. Many EEs come from an asymmetric division of EE precursors, where it seems that Notch signalling distinguishes between the two progeny cells. In the earliest EEs formed in the embryonic midgut, Notch signalling induces a bHLH-Orange TF, Hey, whose expression and function we are investigating in collaboration with IMBB Staff Scientist Dr. Maria Monastirioti.
Later in the adult midgut, intestinal stem cells (ISCs) replenish damaged/aged enterocytes and enteroendocrine cells. The behaviour of ISCs is very plastic in response to genetic and environmental perturbations. In homeostasis they divide slowly to generate "enteroblasts", that will mature to absorptive enterocytes when needed. Inflammatory signals stimulate ISCs to divide more rapidly and result in dysplastic intestines with accumulated enteroblasts. We are studying how chromatin modifications influence ISC behaviour in homeostasis and upon inflammation. [More]