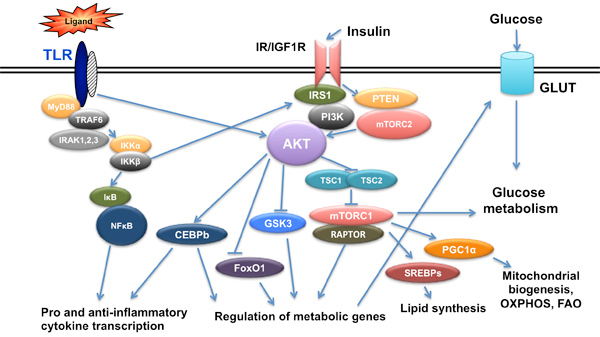
Metabolic and epigenetic mechanisms regulating macrophage responses; the role of insulin signaling and Akt kinases.
We have recently shown that macrophages become insulin resistant and obtain an M2-like phenotype with distinct TLR responses, describing a state of trained immunity. We are currently investigating the epigenetic mechanisms involved in macrophage training in the context of either genetically-induced insulin resistance (Akt2 deficient or IGF1R deficient macrophages) or macrophages derived from diet-induced insulin resistant mice. The interplay between epigenetic alterations at the level of histone modifications and cell metabolism is being investigated. The role of autophagy and phagocytosis in the context of insulin resistance-trained macrophages is also analyzed.
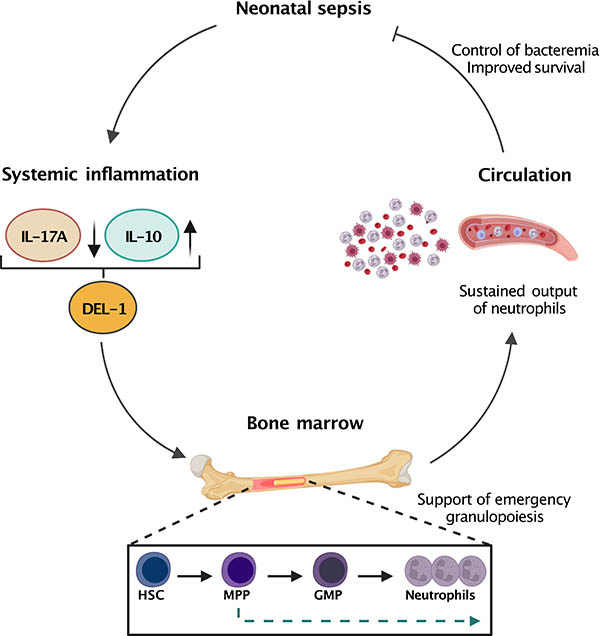
Understanding differences between adult and neonatal innate immune responses
Newborns rely on innate immunity to combat pathogens prior to maturation of the adaptive immune system, yet innate immune responses are still immature and not always effective. Thus, neutrophil recruitment at the site of inflammation is not efficient and the capacity of neonatal macrophages to eliminate bacteria is not as efficient as it is in adult cells. We are currently investigating differences between adult and neonatal innate immune responses in the context of neutrophil recruitment and the role of Del-1 protein during polymicrobial sepsis. We are also studying the role of autophagy as a mechanism of pathogen clearance in neonates and the role of Akt kinases in this context.
The crosstalk of gut microbiome and innate immune responses and the role of dietary and metabolic products
The gut microbiome affects innate immune responses in metabolic and inflammatory diseases. Our group analyzes the impact of insulin resistance-trained macrophages in shaping the gut microbiome using genetically-induced insulin resistant macrophages. We study the impact of nutritional products and metabolites on the gut microbiome and how these affect macrophage responses and inflammatory disease development. We have identified algae-derived terpenes with anti-inflammatory activity. Our cell culture platform screens isolated compounds from marine organisms for efficacy in in vivo models of inflammatory diseases (e.g., obesity/type 2 diabetes, inflammatory bowel disease).
Regulation of macrophage responses in the context of critical illness and sepsis and the role of insulin signaling
Critically ill and septic patients enter a state of trained immunity, characterized as endotoxin tolerance or immunoparalysis. Akt kinases and miRNAs play a key role in regulating endotoxin tolerance. Insulin is continuously infused in critically ill patients, and we are currently investigating the role of insulin oscillatory signaling and insulin resistance in regulating macrophage responses and immunoparalysis, using mouse models and ex-vivo studies from patient samples.
Regulation of adipocyte function and their contribution in metabolic inflammation
Metabolic inflammation is controlled by a complex interaction between adipocytes and immune cells in adipose tissue. Macrophages and adipocytes play central roles in this crosstalk, sharing biological properties. We investigate how nutritional metabolites affect adipocyte differentiation and function, and the role of cytokines and Akt signaling in these processes, especially in metabolic inflammation and obesity.
Circulating ncRNAs as biomarkers of inflammatory and cardiovascular diseases
Circulating ncRNAs are promising biomarkers for disease diagnosis. Our group has identified miRNAs as contributors to inflammatory disease pathologies. In collaboration with Prof. D. Kardassis, we investigate the potential of ncRNAs as biomarkers for cardiovascular diseases, focusing on their differential expression at various stages of coronary disease.




