Current Research Interests :
- Function and regulation of the transcriptional repressor ERF
- Development of ERF activators for therapy approaches
Our long standing interest is the signal regulated transcriptional control in development and disease. We are particularly interested in the ETS gene regulation in response to RTK cascades. For the last several years we have explored multiple facets of the ERF transcription factor as a paradigm of signal-regulated transcriptional control. Taking advantage of the people and the expertise present in our Institute we employ biochemistry, molecular biology, developmental biology, structural biology, stem-cell biology and genome-wide analysis approaches, to tackle the relevant questions.
Our work led to the hypothesis that signaling pathways also signal quantitatively their inactive state through inhibitors/repressors.
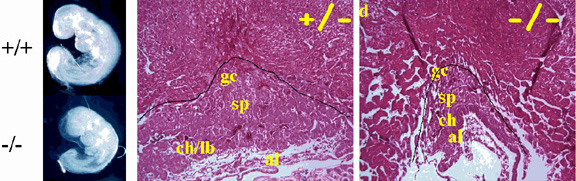
The last several years, we have focused our efforts on ERF a transcription factor of the ets-oncogene family. In contrast to the other ets genes ERF is a transcriptional repressor and can act as a tumor suppressor. We studied the mechanism of its regulation by Erks, and established that a unique protein interaction determines its phosphorylation. We determined that this phosphorylation leads to its regulation by sub-cellular compartmentalisation. We studied its function as a tumor/growth suppressor and we established that Erf can repress cell proliferation via an Rb-dependent pathway and that specific non Erk-regulated mutations can be effective against ras-induced tumorigenesis. We also studied its function in the context of the whole animal through transgenic mice and found that the loss of Erf leads to embryonic lethality as a result of extensive proliferation and defective differentiation of the trophoblast stem cell. We established a role of Erf in the breast cells epithelial-to-mesenchymal-transition (EMT) and identified targets that may mediate the Smad-independent TGFbeta signaling in these cells. We finally showed that Erf haploinsufficiency can cause cranisynostosis in humans and mice.
In all these case decreased/lost ERF, recapitulated some aspects of RTK activation supporting the hypothesis that signaling pathways actively signal their inactivity too.
Our ongoing studies in fundamental research focus on the mechanisms by which ERF affects mesenchymal, hematopoietic, neuronal and trophoblast stem cells as well as mammary epithelial cell growth and differentiation. Our translational research focuses small molecule that could increase the nuclear localization of ERF and thus reverse the adverse phenotypes of ERF haproinsufficiency, and possibly of FGFR activation, in craniosynostosis and other bone growth defects.